Prevalence of Nasopharyngeal Carriage in Children Hospitalized with Pneumonia

Streptococcus pneumoniae bacteria (pneumococcus) is a major cause of disease and disability worldwide, and is vaccine-preventable. In 2015, the Ministry of Health of Nepal began providing a 10-valent pneumococcal conjugate vaccine to infants (PCV10) as the vaccine was introduced into its routine infant immunization schedule.
Estimating the impact of PCV on carriage in pneumonia cases
The pneumococcus is a bacterium that is often found in the nasopharynx (i.e., the back part of the nasal cavity) of children and adults without causing disease or symptoms. Pneumococcal disease occurs when the bacteria move from this harmless location—nasopharyngeal carriage—to other more vulnerable parts of the body, including the lungs (where it causes pneumonia).
More than 90 distinct pneumococcal serotypes have been identified throughout the world, however only a small number of these serotypes cause the majority of disease in infants. The vaccine Nepali children receive, PCV10, protects against ten pneumococcal serotypes. The Inpatient Carriage (INCA) study is measuring whether the ten vaccine serotypes are less likely to be found in children hospitalized with pneumonia following the introduction of PCV10, as an indicator of the vaccine's effectiveness. The study is also measuring:
- The number of bacteria present in the nasopharynx (i.e. carriage density) of each child with pneumonia, and
- Whether serotypes absent from the vaccine are more likely to be carried after vaccine introduction.
Since 2014, our team has collected and examined nasopharyngeal specimens from every child between the ages of two months and 14 years admitted with a clinical diagnosis of pneumonia to Patan Hospital, a major teaching hospital in Kathmandu.
Preliminary Results: Reduced vaccine-type carriage in children hospitalized with pneumonia
Between March 2014 and end of December 2019 a total of 2051 children were enrolled into the Inpatient Carriage Study: 210 children were enrolled in 2014, 267 children in 2015, 461 children in 2016, 380 children in 2017, 411 children in 2018 and 322 children in 2019.
Since the introduction, there are indications that the PCV10 vaccine is reducing pneumococcal carriage in pneumonia patients. Among children between two months and two years—those eligible to have received the vaccine—admitted to hospital with pneumonia, the percentage of patients with carriage of pneumococcal serotypes targeted by PCV10 has fallen from 39% in the pre-vaccine era to 31% in the post-vaccine era.
After the introduction of PCV10 in 2015, children hospitalized with pneumonia seemed less likely to be carrying types of bacteria contained in the vaccine.
However, part of this initial decline in the proportion of hospitalized pneumonia cases with carriage could be due to year-to-year variability in circulating serotypes. Additional years of data collection will help clarify the effect of PCV10 on carriage in children hospitalized for pneumonia.
Fig. 2
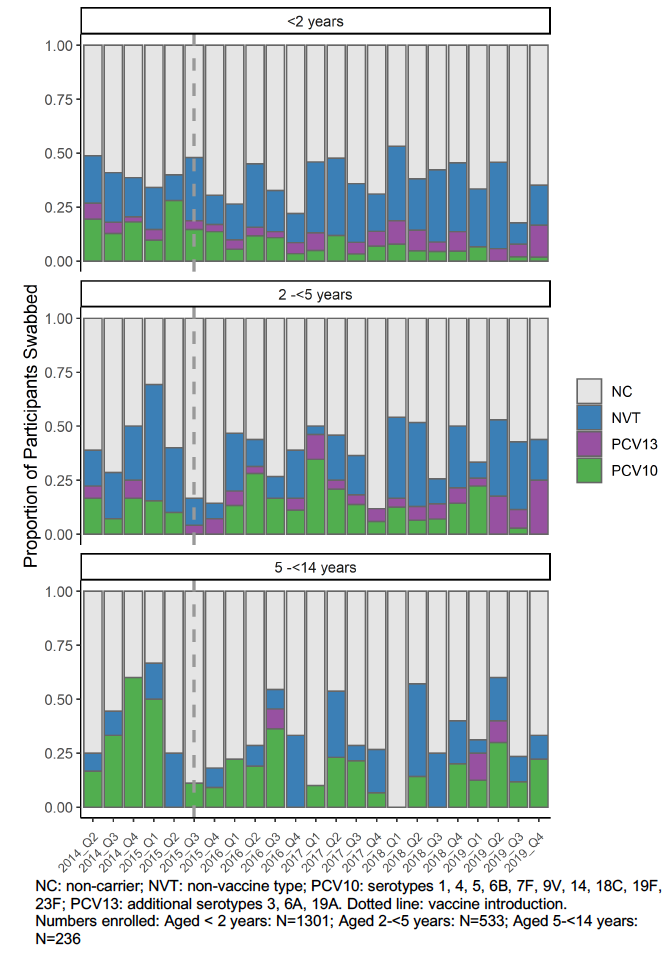
In the three years following introduction of the vaccine, a decline in carriage of vaccine serotypes can be observed for children less than 2 years of age. For older cohorts (2 to < 5 years of age and 5 to <14 years) numbers of pneumonia cases are lower and the data are more variable. A reduction in PCV10 serotypes is observable in 2019 for those aged 2 to < 5 years. No reduction is seen in those aged 5 to > 14 years.
Analysis of this study is ongoing. For more information, contact our study coordinator.



